We are applying our expertise in genomic profiling to identify elusive cancer-related genetic changes and new biomarker targets across many cancer types. Our advanced cancer genome analysis helps researchers and biopharmaceutical partners accelerate new precision medicine development.
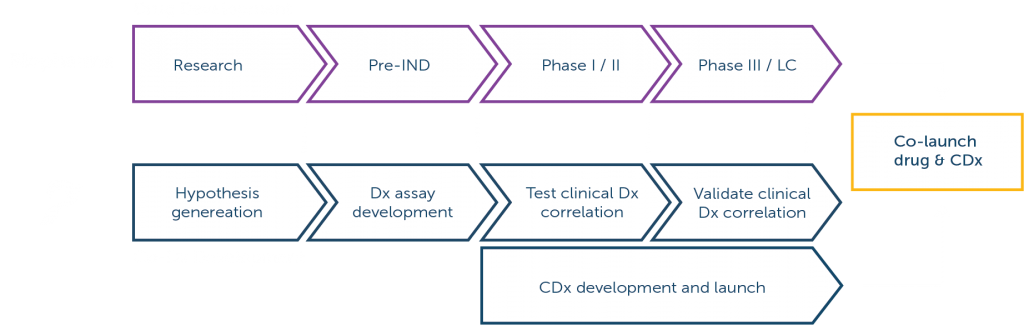
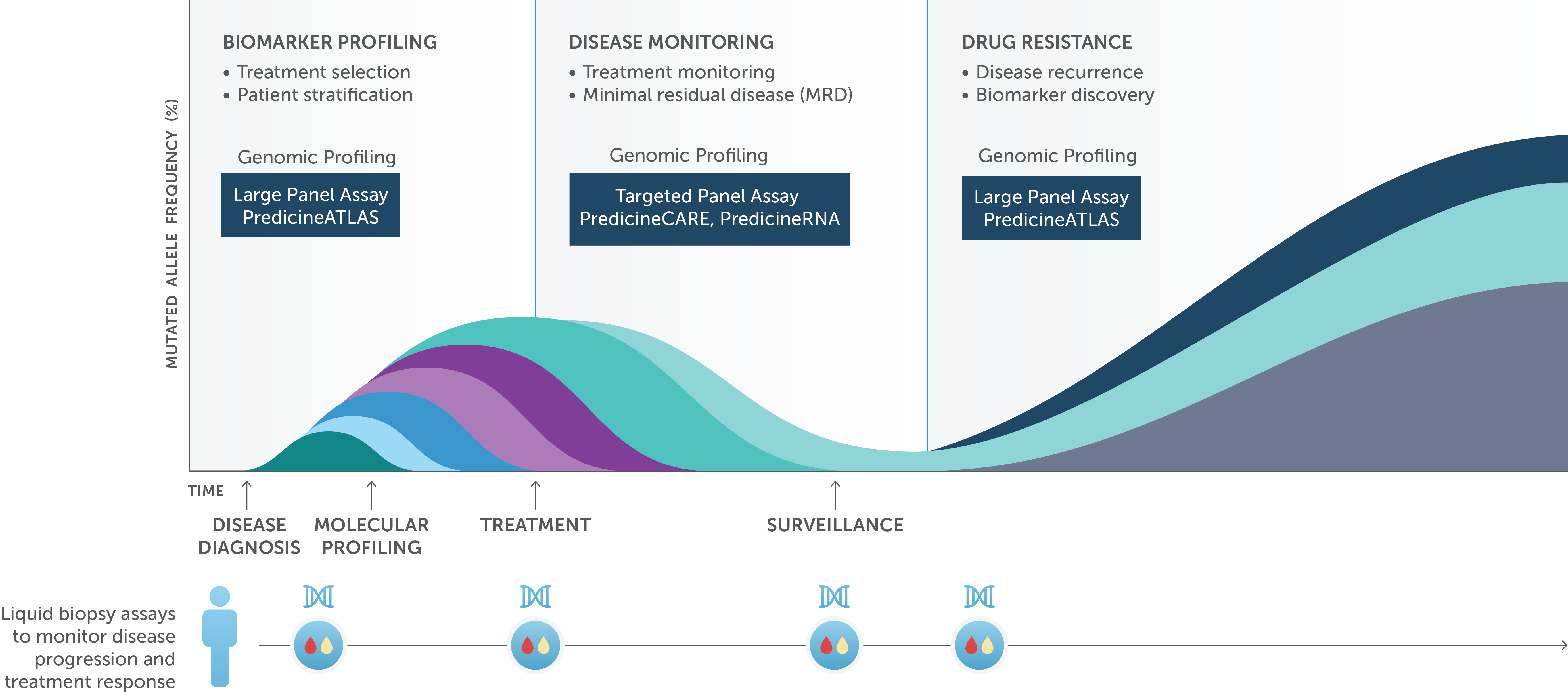
We offer a portfolio of comprehensive genomic profiling assays for precision medicine development.
| ALL SOLID TUMORS | HEMATOLOGIC MALIGNANCIES | ||||
|---|---|---|---|---|---|
| COMPARE | PredicineATLAS™ | PredicineCARE™ | PredicineRNA™ | PredicineMRD™ | PredicineHEME™ |
| OVERVIEW | Broadest gene coverage | Pan-cancer panel (includes guideline-recommended genes) | Panel for known or novel gene fusion / splice variant | Targeted panel for minimal residual disease assessment | Panel for hematologic malignancies |
| NUMBER OF GENES | 600 (DNA) | 152 (DNA) | 88 (RNA) | Indication specific | 106 (DNA) |
| SPECIMEN TYPE | Blood Urine Tissue | Blood Urine Tissue | Blood Tissue | Blood Urine | Blood Bone marrow aspirate |
| TURNAROUND TIME | 10 Days | 10 Days | 10 Days | 10 Days | 10 Days |
| TMB | ✓ | — | — | — | — |
| MSI | ✓ | ✓ | — | — | — |
| MMR | ✓ | ✓ | — | — | — |
| HRD | ✓ | ✓ | — | — | — |
| REPORT FEATURES | SNVs INDELs CNA CNL Rearrangements | SNVs INDELs CNA CNL Rearrangements | RNA fusions RNA splice variants | SNVs INDELs CNA CNL Rearrangements Methylation Chromosomal abnormalities | SNVs INDELs CNA Rearrangements |
| DEPTH | 20,000x | 20,000x | — | 100,000x | 20,000x |
| SENSITIVITY | 0.25% down to 0.05% | 0.25% down to 0.05% | 10 copies | 0.01% | 0.25% down to 0.05% |
| VERSIONS | CLIA RUO | CLIA RUO | RUO | RUO | RUO |
| WHOLE EXOME SEQUENCING | ✓ About cfWES | ✓ About cfWES | — | — | — |
| DETAILS | About PredicineATLAS™ | About PredicineCARE™ | About PredicineRNA™ | Contact us | About PredicineHEME™ |
We provide harmonized assays supported by unified quality management systems and workflow in our network of CLIA-certified and CAP-accredited facilities in U.S. and China to support cross-border clinical studies.
Ready to streamline your precision oncology clinical research programs? Submit your information to speak to a team member.