• Detects gene deletion and mono- vs. bi-allelic loss of function
• CLIA-certified assay for blood and urine
• Adopted by leading biopharmas in clinical trials




*CLIA-validated assay requires two tubes of 10mL blood
PredicineCARE™ provides important insights to a tumor’s genomic landscape through blood, urine, and tissue. It analyzes tumor-associated genetic aberrations to provide comprehensive molecular insights into a patient’s tumor biology to help determine whether targeted therapies are treatment options.
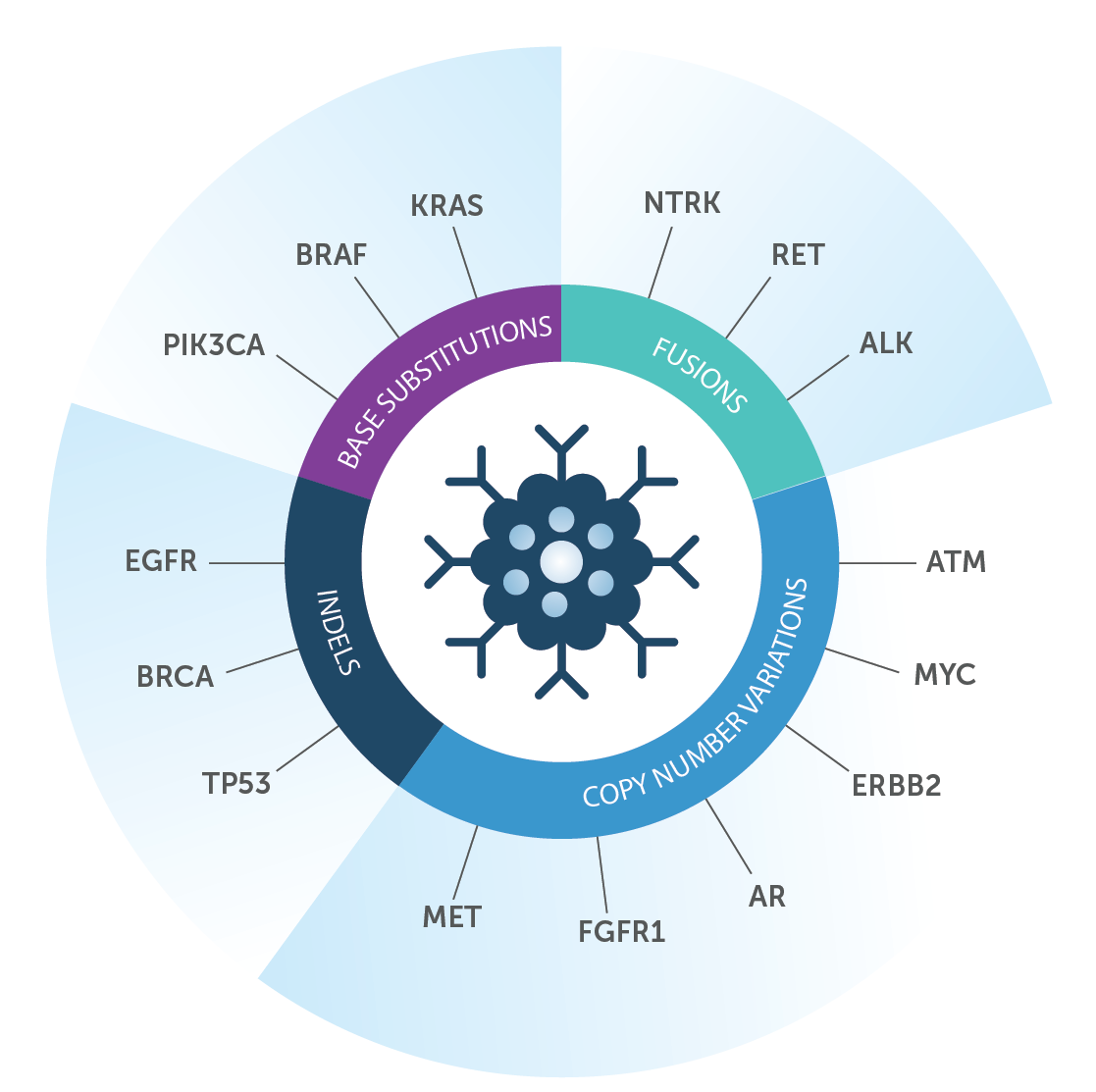
The assay analyzes genes directly linked to specific cancers by well documented scientific research. It is designed to identify all main classes of actionable genomic alterations, including base substitutions, insertions and deletions, copy number alterations, selected fusions and homologous recombination deficiency. See figure below.
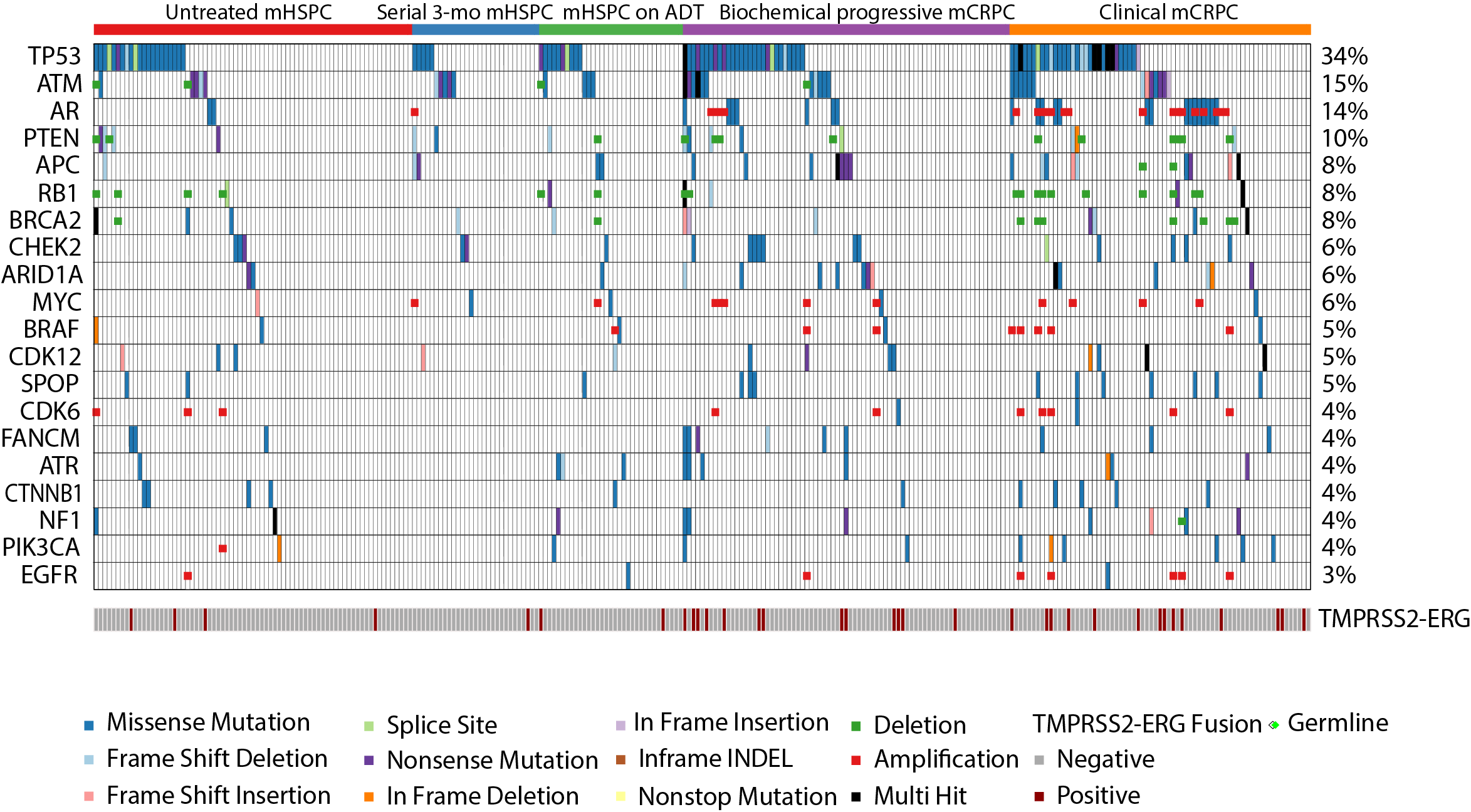
Manish Kohli et al. Clinical and genomic insights into circulating tumor DNA-based alterations across the spectrum of metastatic hormone-sensitive and castrate-resistant prostate cancer, EBioMedicine, Volume 54, 2020, 102728
We offer pilot program grants to select biopharma and academic partners to empower translational research and clinical studies. To initiate a study, contact us via the form below.
If you have any questions or need assistance, complete this form and we will respond within 24 hours.